Salmonella Carriage by Herring Gulls in the Clyde Area of Scotland in Relation to their Feeding Ecology
P. Monaghan, C. B. Shedden, K. Ensor, C. R. Fricker* and R. W. A. Girdwood*Department of Zoology, University of Glasgow, Glasgow G12 8QQ, and
*Department of Bacteriology, Stobhill General Hospital, Glasgow G21 3UW
Summary
(1) Between February 1982 and February 1984, 2985 herring gulls, captured at refuse tips in the Clyde area, were examined for the presence of salmonellae in their faeces; 9.2% of those examined in the breeding season were found to be carrying salmonellae, and 9.8% during the non-breeding season. The most common serotypes isolated were Salmonella virchow and S. typhimurium.(2) The proportion of these herring gulls carrying salmonellae was significantly positively correlated with the incidence of salmonellosis in the human population in the same area at the same time, and presumably reflects the level of environmental contamination.
(3) There were no statistically significant differences in carriage rates between different age classes of herring gull, but during the non-breeding season, there was a highly significant difference between the sexes. The rate of female carriage at this time was more than double that of males, and appears to reflect differences in their feeding ecology.
(4) A further 163 herring gulls obtained from breeding colonies were similarly examined. These had a significantly higher carriage rate than those examined at refuse tips at the same time, indicating that other kinds of feeding areas may give rise to more frequent infection of gulls.
(5) There was no evidence that infection with salmonellae affected the health of the gulls concerned. Such pathogen carriage by gulls, when coupled with their considerable powers of dispersal, may give rise to potential public health problems, particularly when gulls are roosting on potable water supplies.
Introduction
In recent years, concern has been mounting over the role of Larus gulls in the dissemination of human pathogens. In addition to their traditional coastal haunts, these birds now feed at refuse tips and sewage outfalls, roost at night on inland water reservoirs and by day on pastureland, and nest on inhabited buildings. This brings them into proximity with man, his pathogens, domestic animals, foodstuffs and water supplies. Moreover, the present century has seen considerable population increases in all five species of gull which commonly breed in Britain. In particular, the herring gull (Larus argentatus Pontoppidan) has been doubling in numbers every 6 years for much of this century (Chabrzyk & Coulson 1976), and its present population in Britain is in excess of two million. This change in status is reflected in the increased incidence of urban nesting herring gulls and the increasing numbers found inland (Hickling 1977; Monaghan & Coulson 1977). In view of these population changes, such problems as these birds create are likely to increase, and, coupled with their feeding habits and considerable powers of dispersal, it is not surprising that they have been implicated in the spread of infection (Parsons & Duncan 1978; Monaghan 1980; Coulson, Butterfield & Thomas 1983).
Numerous salmonella serotypes have been isolated from the faecal material of British gulls (e.g. Williams, Richards & Lewis 1976; Johnston, Maclachlan & Hopkins 1979; Fenlon 1981; Reilly et al. 1981; Benton et al. 1983; Butterfield et al. 1983). These bacteria are among the causative agents of gastro-intestinal infections in man and domestic animals; over two and a half thousand cases of such infection in humans are reported annually in Scotland, which is likely to represent only a small fraction of the number occurring (Vernon 1977).
Since these birds have been implicated in the spread of infection to man and his domestic animals (e.g. Jones, Smith & Watson 1978; Johnston, Maclachlan & Hopkins 1979) it is clearly important that we have a realistic picture of the proportion of gulls carrying salmonellae, and the extent to which this relates to their feeding ecology. This information, coupled with an understanding of the movement patterns and behaviour of the birds, will enable their role in the dissemination of these pathogens to be assessed, and the possible hazards which they pose to public health to be evaluated. This paper presents the results of a detailed study of the proportion of herring gulls of known age and sex carrying salmonellae in the Clyde area of Scotland. The results reported here form part of a wider study encompassing all of Scotland, details of which are reported elsewhere (Girdwood et al. in press).
Methods
It was essential to this study that gulls were caught in large numbers at different localities. This was done by cannon-netting flocks of gulls at feeding sites, predominantly refuse tips, with up to 350 gulls being caught at one time. Over 8000 herring gulls were caught at eight refuse tips in the Clyde area (Fig. 1) by this method between October 1978 and February 1984. During the period February 1982 to February 1984, 2985 of these herring gulls were examined for the presence of salmonellae in their faeces. In addition, a further 163 herring gulls, obtained dead from culls during 1982 and 1983 at the breeding colony on Horse Island in the Firth of Clyde (carried out by the Royal Society for the Protection of Birds during control measures), were examined for the presence of salmonellae in the gastro- intestinal tract.
In culled gulls the entire gut was cultured for the isolation of salmonellae, whereas with captured birds faecal samples were obtained by cloacal washing. This latter procedure identifies 15-20% fewer carriers than does the gut culture (Girdwood et al. 1985). Data from culled birds are treated separately in the analyses. Full details of the microbiological methods used in the isolation of salmonellae from this material are given in Fricker, Girdwood & Munro (1983) and Fricker & Girdwood (1984). The nomenclature used for the Salmonella spp. isolated follows that recommended by the WHO Collaborating Centre for Reference and Research on Salmonella (Anon. 1984).
The captured birds were aged in the hand up to the fifth year of life by plumage characteristics (see Grant (1982) for details) and divided into first years, intermediates (2-4 years) and adults (5th year and older) for the purposes of analyses. Birds aged in the field were divided, for ease of identification, into immatures (lst and 2nd years, i.e. basically brown birds) and 3+ years (i.e. birds showing clear signs of adult plumage). Salmonella carriage by herring gulls is considered here in relation to both the Clyde area breeding population of these birds and the wintering population. Throughout this paper the breeding season is taken as April to July, and the non-breeding or wintering period as October to February.
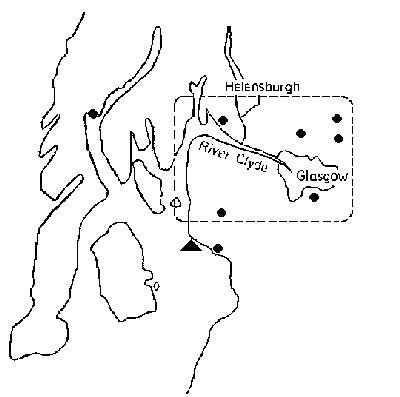
FIG. 1. The Clyde area to which this study refers. The locations of the eight refuse tips at which gulls were caught (circles) and of Horse Island (triangle), the breeding colony from which the culled birds were obtained, are shown. The dotted line ( ---- ) indicates the area within which all nocturnally roosting herring gulls, and those feeding at refuse tips, were counted.
Standard biometric measurements (see Coulson et al. (1984) for details) were taken from 61% of the captured birds. Live gulls from which biometric measurements were taken were sexed using the combined head and bill measurement which discriminates between the sexes with an accuracy of c. 95% (Coulson et al. 1983a); dead gulls were sexed by dissection. The live birds were released shortly after capture, having been individually marked using a combination of dye marking and colour ringing; this permitted subsequent field observations of relevant movements and behaviour of individuals. The birds were also given a numbered metal ring, as a result of which recoveries of dead birds were reported to us through the British Trust for Ornithology Ringing Scheme.
Counts of birds feeding within the study area were made from inside a vehicle, using binoculars; roosting birds were counted either as they came into roosts at dusk, or after dusk using an image intensifier. In order to estimate the proportion of herring gulls present in the area feeding at refuse tips, regular weekly visits were made to all refuse tips and all nocturnal roosting sites within a delimited area (Fig. 1) throughout one winter from November to January, and the number of herring gulls counted. In addition, herring gulls feeding at the refuse tip at Helensburgh were observed all day on each of five consecutive weekdays once per month for 12 months. These data are used here to give an estimate of the extent to which individual herring gulls used a single refuse tip at different times of year. The other tips were visited in a regular monthly circuit. All colour-ringed herring gulls present on each visit to a tip were recorded. Movements of herring gulls were therefore documented by means of repeated sightings of individually marked birds and recoveries of dead birds by ourselves, co-workers and members of the public.
Results
Salmonella carriage by herring gulls in the Clyde areaThe overall incidence of salmonellae found in herring gulls caught at refuse tips during the breeding season was 9.2% (588 birds examined) and 9.8% during the non-breeding season (1433 birds examined).
TABLE 1. The salmonella serotypes isolated from herring gulls in the Clyde area.
The number of positives of each serotype is given, plus the percentage of the
total number of gulls (3148) examined which carried each serotype
Serotype Number of isolates Per cent of herring gulls examined
agona 5 0.16
anatum 2 0.06
braendenburg 1 0.03
bredeney 26 0.83
hvittingfoss 1 0.03
haardt 4 0.13
hadar 6 0.19
heidelberg 2 0.06
indiana 4 0.13
infantis 10 0.32
java 1 0.03
livingstone 4 0.13
mbandaka 10 0.32
montevideo 7 0.22
newport 12 0.38
panama 1 0.03
saint-paul 2 0.06
schwarzengrund 1 0.03
stanley 9 0.29
typhimurium 90 2.86
virchow 186 5.91
4,12:d- 1 0.03
6,7: r- 1 0.03
Table l lists the twenty-three salmonella serotypes isolated from the 317 positive faecal samples from herring gulls examined during this study. The most common serotypes were Salmonella virchow and S. typhimurium, which accounted for 48 and 23%, respectively, of the total number of salmonella isolations in the Clyde area. The proportion of herring gulls caught at refuse tips in the Clyde which were positive for salmonellae is strongly correlated with the incidence of salmonellosis in the human population in the same area at the same time (Fig. 2). Using gulls from which biometric measurements were taken, and which could therefore be sexed, Table 2 shows the proportions of herring gulls of each age and sex class, caught at the tips, which were found to be carrying salmonellae at different times of year. Within a season, there were no significant differences in the carriage rates between the age classes for either males or females. When these data were examined in relation to seasonal differences, there was a highly significant difference in the carriage rates of females (X2 = 14.23, d.f. = 1, P < 0.001, all age classes combined), the carriage rates during the non-breeding period (22%) being three times that recorded during the breeding season (7%). There was no significant difference in the carriage rates of males, which remained around 10% throughout the year (overall value for all age classes combined). The incidence of salmonella carriage in the tip-caught birds did not differ between the sexes during the breeding season. During the non-breeding season however, the difference between the sexes was significant (Table 2, X2 = 8.03, d.f. = 1, P < 0.01, all age classes combined); the rate of female carriage (22%) was more than double that of males (10%). Table 3 gives the carriage rates of adult breeding herring gulls culled at Horse Island during the incubation period in May. As found for adults caught at refuse tips during the same period, there was no difference between the sexes in the carriage rate; however, the carriage rates in the culled birds were considerably higher than those found at refuse tips (Tables 2 & 3).
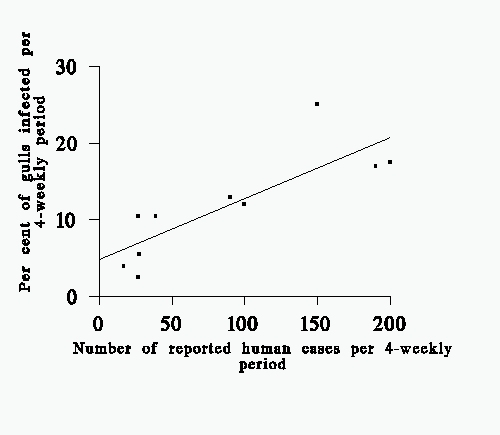
FIG. 2. The proportion of herring gulls (%) per 4-weekly period which were found to be infected with salmonellae, and the number of human cases during the same periods in the same area. (Data from humans supplied by the Communicable Diseases (Scotland) Unit, Ruchill Hospital). The two are significantly correlated (r= 0.78, d.f. = 9, P < 0.01). The line drawn was fitted by least squares regression (y = 0.074x + 5.44).
TABLE 2. The proportion of male and female herring gulls caught at refuse tips in the Clyde area during the breeding and non-breeding seasons, which were found to be +ve for salmonella carriage
Period Age (year) Number +ve Number -ve Percent +ve
Males
April-July Adult 6 67 8.2
2-4 7 64 9.9
1st 8 32 20.0
October-February Adult 10 111 8.0
2-4 4 33 10.8
1st 7 36 16.3
Females
April-July Adult 6 57 9.5
2-4 3 63 4.6
1st 1 26 3.7
October-February Adult 18 73 20.0
2-4 9 21 30.0
1st 8 31 20.5
Even when the Horse Island data are corrected to allow for the difference in isolation rates between the two sampling methods (Table 3), the difference between the adult herring gulls caught at colonies and those adults caught at tips remains highly significant for both sexes (males X2 = 9.13, d.f. = 1, P < 0.01; females X2 = 10.97, d.f. = 1, P < 0.001). Body weights of gulls positive for salmonellae were compared with those that were negative, taking account of differences in body size and seasonal weight changes (Coulson et al. 1983b). There was no indication of any loss of body condition in those gulls found to be carrying salmonellae. Recovery rates of dead birds were also examined, and there was no evidence to suggest that herring gulls which carried salmonellae had higher mortality rates, or travelled shorter or longer distances, than those which did not.
TABLE 3. The salmonella carriage rates of breeding herring gulls obtained from culls on Horse Island in the Firth of Clyde in 1982 and 1983. The figures in parentheses represent the proportion of positives and negatives corrected to take account of differences in isolation techniques between these birds and live birds caught at tips during the same period; this enables comparisons between the two groups
Number Number Per cent Corrected
+ve -ve +ve per cent +ve
Males 18 (14) 31 (35) 36.7 (28.6)
Females 30 (25) 46 (51) 39.5 (32.9)
The use of refuse tips by herring gulls in the Clyde area
Greater numbers of herring gulls are found at the refuse tips in winter than in summer, as can be seen from Fig. 3, which shows the mean number present at a refuse tip near Helensburgh in the Clyde area throughout the year. Other tips in the area show the same pattern, with large numbers, particularly of adults present in the winter months (Shedden, 1983). Data based on the weekly observations at Helensburgh tip showed that individual birds were more likely to be present at this particular site on more than 1 day per week in winter than in the breeding season (Table 4); there was no difference between age or sex classes in this respect.
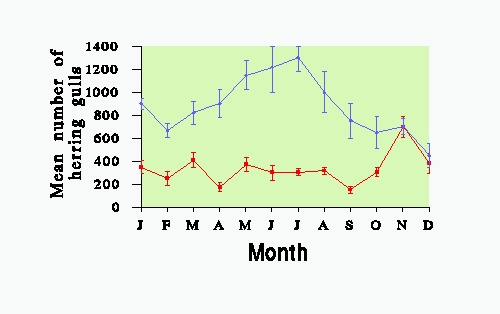
FIG. 3. The mean (+ 1 S.E.) number of herring gulls present at Helensburgh refuse tip throughout the year. (RED), the numbers of 1st + 2nd years; (BLUE), the older age classes.
The counts of the numbers of herring gulls roosting in the area shown in Fig. 1, in comparison with the numbers counted feeding at refuse tips in the same area showed that, on average, 77% of the herring gulls roosting in the area were to be found feeding at refuse tips on weekdays during the winter period (Table 5). While there is clearly not a closed population roosting and feeding in this area, these data demonstrate that refuse tips are a major food supply for herring gulls wintering in the Clyde area.
TABLE 4. The number of individually marked herring gulls present on I day per week and more than I day per week at Helensburgh refuse tip, during the breeding and non-breeding seasons. A greater proportion of the birds was present at the tip more than I day per week outside the breeding season (X2 = 4.9, d.f. = 1. p < 0.05)
Period Number present 1 day week-1 Number present > 1 day week-1 April-July 124 (62.7%) 74 (37.3%) October-February 162 (52.3%) 148 (47.7%)
TABLE 5. The number of herring gulls roosting overnight within a delimited area (see Fig. 1) and the number within this area found feeding at refuse tips. The proportion of the roosting population feeding at tips is also shown (see Shedden (1983) for further details)
November December January
Number roosting 8500 13950 10000
Number feeding at tips 7250 8330 8660
Per cent feeding at tips 85 60 86
Difference between the sexes in the use of refuse tips
Of 1520 adult herring gulls caught at tips in the Clyde area during the non- breeding season, 64% were female; similarly 64% of 464 adult herring gulls caught in the same area during the breeding season were female. Amongst the younger age classes, females predominated at tips during the non- breeding season (61% of 790 1-year-olds and 58% of 566 2-4-year-olds), but during the breeding season the sex ratio in these age classes was almost equal (48% of 190 1st year and 47% of 382 2-4-year-olds were female). As stated above (Table 4), both sexes visited a particular tip more often in winter than in summer, and a greater number of herring gulls was present at tips on any one day in winter (Fig. 3). The high proportion of females found at tips could be due to fewer males than females being present in the Clyde area overall, or to males using other kinds of feeding site. Observations on the sex ratio of herring gulls at different types of feeding site support the latter suggestion. Of twenty-four adult herring gulls caught behind trawlers in the Clyde area during the non-breeding season in 1981, sixteen (67%) were male, which differs significantly from the sex ratio in catches at tips in the Clyde area at the same time of year X2 = 8.32, d.f. = 1, P < 0.01). Herring gulls do move regularly between different kinds of feeding sites, and our sightings of birds marked at tips also indicate that males are more likely to follow fishing boats than females. Three times as many marked males as females were subsequently seen feeding around fishing boats (twenty-one males and seven females), a highly significant difference from the proportion expected based on the numbers marked (3101, of which 47% were male; X2 = 9.07, d.f. = 1, P < 0.01). It thus appears that, while refuse tips are clearly utilized extensively by herring gulls in the Clyde area, particularly in winter, male herring gulls use them comparatively less often than females.
Dispersal of herring gulls
Clearly, a comparatively high proportion of the herring gulls in the Clyde area carry salmonellae. During the winter period, between twenty-five and thirty thousand herring gulls roost overnight in the area, and the breeding population is of the order of five and a half to six thousand. Herring gulls are highly mobile and undertake both seasonal movements to and from the breeding colonies in spring and autumn, respectively, and day-to-day movements to and from the feeding sites. A proportion of the breeding population in the Clyde area also overwinters in that area, while others move south at the end of the breeding season. There is a corresponding movement of herring gulls into the Clyde area in winter from the breeding colonies to the north and west. The locations of these colonies are shown in figure 4a. The range of movements of younger birds, which are not tied to breeding colonies, is shown in figure 4b. These data demonstrate the extent of the movements of which these birds are capable, involving distances of hundreds of kilometresin a short time. Our sightings records show both immatures and adults moving over 250 km in less than a week, clearly minimal estimates.
Discussion
The data presented in this paper show that a high proportion of herring gulls feeding at tips in the Clyde area (c. 10%) are carrying salmonellae. The carriage rate in the Clyde area is generally higher than that which we have found elsewhere in Scotland using the same methods, and such regional differences appear to be related to human population density (Girdwood et al. in press). Furthermore, the salmonella serotypes present in gulls and humans are similar, with S. typhimurium and S. virchow being amongst the most common in both groups, and these serotypes are also those most commonly isloated from environmental sources (Table 1; Anon. 1982, 1983). Since gulls typically carry only small numbers of salmonellae (Fenlon 1981; Girdwood et al. in press), it is unlikely that they infect humans directly. These data suggest that the close association which now occurs between gulls and man gives rise to the infection of gulls with human pathogens; this could result either from ingestion of infected material at feeding sites, or the drinking of polluted water while roosting on contaminated estuaries. It is likely that, when the degree of contamination of the environment with salmonellae increases, the rate of carriage of these pathogens by gulls increases.
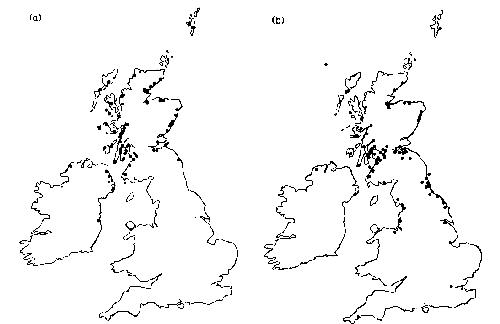
Fig. 4.(a) Locations where marked adult herring gulls, caught in the Clyde area outside of the breeding season, were found to breed. (b) The locations where marked immature herring gulls (<4 years old), caught in the Clyde area, have been seen or found dead. Birds have also been recovered in North Norway, Denmark and Belgium.
The large number of herring gulls feeding in the Clyde area, particularly in winter, make considerable use of food sources supplied from human waste, such as refuse tips and sewage outfalls, which are likely to be contaminated with salmonellae (Durrant & Beatson 1981; Fenlon 1983; Fricker 1984). They appear to be more dependent on these sources than are gulls elsewhere in Scotland since there is no large supply of fish material, their traditional diet, available at fishing ports or around fishing fleets in the vicinity. Landings of fish species from which offal and discards are produced (mainly cod, haddock and whiting) are very small in the Clyde area compared with the North Sea fishing ports on the east coast of Scotland (Fisheries Statistics Unit, D.A.F.S., personal communication). This study has shown that many more herring gulls are present at Clyde tips in winter than in summer, as has been found in other areas (Horton, Brough & Rochard 1983). More than three quarters of the herring gull population wintering in the Clyde area is found feeding at refuse tips. It is more difficult to estimate the proportion feeding at tips during the breeding season. The total population cannot be estimated from roost counts since the adults, plus an unknown proportion of younger birds, roost at the breeding colonies. In addition, counts of birds at tips are less reliable because breeding birds also spend less time loafing at tips, making only short foraging trips to and from the nesting site.
The association between the prevalence of salmonella carriage by gulls and their feeding ecology is further demonstrated by the difference between the sexes in both these respects. Other studies (Spaans 1971; Kihlman & Larsson 1974) have found a preference in herring gulls for the more traditional kinds of feeding areas, rather than refuse tips; the latter are used most when the weather is severe. What food resources are available to herring gulls from the fishing industry in the Clyde appear to be preferentially used by adult males, and the majority of herring gulls present at tips in the area are female. However, the male herring gulls present at tips dominate the females and, when competition is severe, as in winter, males monopolize the best feeding areas of freshly dumped refuse (Monaghan 1980; Greig, Coulson & Monaghan in press). Females tend to concentrate on secondary areas of older refuse, and thus will have a higher intake of more putrid food, which may contribute to their increased salmonella carriage in winter. Furthermore, since the availability of alternative food sources is reduced in hard weather, and there are also more birds in the area in winter, the dependence of females on feeding areas such as tips is likely to be greatest at this time.
No statistically significant differences were found between age classes in this study, though other workers have found that elsewhere 1st year herring gulls are more likely to carry salmonellae than adults, possibly due to their increased use of sewage outfalls for feeding (McDonald & Brown 1974, Butterfield et al. 1983). Individual herring gulls show a degree of specialization in feeding site, particularly during the breeding season (Davis 1975; Sibly & McCleery 1983). Thus samples of herring gulls obtained from culls at breeding sites include birds which have feeding specializations other than refuse tips, such as foraging on the coast, at sea or at sewage outfalls. The high rate of salmonella carriage found in adult herring gulls obtained at breeding colonies, in comparison with those adults obtained at refuse tips at the same time (the majority of which will also be breeding birds), suggests that there may be another source of contamination of gulls other than tips. High incidences of salmonellae have been recorded in gull droppings collected around sewage outfalls (Fenlon 1981, 1983; Fricker 1984) and it is likely that these feeding sites contribute to the higher incidence found in gulls at the Horse Island colony, since, in summer, large numbers of birds from this site are known to frequent sewage outfalls on the nearby Ayrshire coast and also follow sewage sludge boats dumping in the Firth of Clyde (N. Metcalfe personal communication).
There was no evidence in this study that contamination with salmonellae affected the health of the gulls concerned, a similar result to that found elsewhere (Butterfield et al. 1983). Experimental studies with captive gulls have shown that salmonella carriage is generally passive, lasting only a few days and that the gulls are not actively infected (Girdwood et al. in press). It is unlikely that ingestion of contaminated gull faeces on pastureland would lead directly to salmonella infection in livestock, other than in animals rendered susceptible through illness or stress (Spence & Westwood 1978). However, numerous salmonella outbreaks amongst livestock in Scotland have been attributed to infections having been brought into the area by gulls (Reilly et al. 1981). The dispersal powers of gulls are considerable and, particularly during spring and autumn, they undertake rapid and comparatively long range migrations to and from the breeding areas. They could therefore be involved in the dissemination of pathogens over considerable distances within Britain.
The hazards arising from the pollution of potable water supplies by roosting gulls, while potentially more serious, are usually offset by the fact that the water is disinfected before distribution to the public. Gulls feeding at coastal sewage outfalls will mostly roost on the coast rather than on inland reservoirs. However, the distribution of herring gulls inland in winter is greatly influenced by the distribution of refuse tips, and gulls feeding at inland refuse tips tend to use adjacent water storage reservoirs as night roosts (Benton et al. 1983; Horton, Brough & Rochard 1983; Shedden 1983). In some areas of Scotland, rural water supplies receive no disinfection. In this situation, if there is a refuse tip nearby, every effort should be made to safeguard the purity of the supply by preventing gulls from roosting on the water at night, or bathing in it by day. This is especially true in winter since the numbers roosting nocturnally on water are greatest at this time of year.
Acknowledgements
We thank the District Authorities in Strathclyde Region for access to refuse tips, all those who assisted in catching and sighting gulls, the Communicable Diseases Scotland Unit, Ruchill Hospital for providing comparative data on humans and D. Munro for much assistance in the laboratory. Thanks also to N. Metcalfe, J. C. Coulson and J. Butterfield for comments on an earlier draft of the manuscript. This work was supported by a grant from the Scottish Home and Health Department.
References
Anon. (1982). Communicable Diseases in Scotland. Annual Summary of Salmonellosis 1982. CDS Unit, Ruchill Hospital, Glasgow. Anon. (1983). Communicable Diseases in Scotland. Annual Summary of Salmonellosis 1983. CDS Unit, Ruchill Hospital, Glasgow. Anon. (1984). Antigenic Formulae of the Salmonella. WHO Collaborating Centre for Reference and Research on Salmonella, Institut Pasteur, Paris. Benton, C., Khan, F., Monaghan, P., Richards, W. N. & Shedden, C. B. (1983). The contamination of a major water supply by gulls (Larus sp.). A study of the problem and remedial action taken. Water Research. 17, 789-798. Butterfield, J., Coulson, J. C., Kearsey, S. V., Monaghan, P., McCoy, J. H. & Spain, G. E. (1983). The herring gull Larus argentatus as a carrier of salmonella. Journal of Hygiene, Cambridge, 91, 429-436. Chabrzyk, G. & Coulson, J. C. (1976). Survival and recruitment in the herring gull Larus argentatus. Journal of Animal Ecology, 45,187-203. Coulson, J. C., Butterfield, J. & Thomas, C. (1983). The herring gull Larus argentatus as a likely transmitting agent of Salmonella montevideo to sheep and cattle. Journal of Hygiene, Cambridge, 91, 437-443. Coulson, J. C., Thomas, C. S., Butterfield, J. E. L., Duncan, N., Monaghan, P. & Shedden, C. (1983a). The use of head and bill length to sex live gulls (Laridae). Ibis, 12S, 549-557 Coulson, J. C., Monaghan, P., Butterfield, J. E. L., Duncan, N., Shedden, C. & Thomas, C. S. (1983b). Seasonal changes in the Herring Gull in Britain: weight, moult and mortality. Ardea, 7, 235-244. Coulson, J. C., Monaghan, P., Butterfield, J. E. L., Duncan, N., Ensor, K., Shedden, C. & Thomas, C. S. (1984). Scandinavian Herring Gulls wintering in Britain. Ornis Scandinavica, 15, 79-88. Davis, J. W. F. (197S). Specialization in feeding location by herring gulls. Journal of Animal Ecology, 44, 795-804. Durrant, D. S. & Beatson, S. H. (1981). Salmonellae isolated from domestic meat waste. Journal of Hygiene, Cambridge, 86, 259-264. Fenlon, D. R. (1981). Seagulls (Larus spp.) as vectors of salmonellae: an investigation into the range of serotypes and numbers of salmonellae in gull faeces. Journal of Hygiene, Cambridge, 86,195-202. Fenlon, D. R. (1983). A comparison of salmonella serotypes found in the faeces of gulls feeding at a sewage works with serotypes present in the sewage. Journal of Hygiene, Cambridge, 91, 47-52. Fricker, C. R. (1984). A note on salmonella excretion in the black-headed gull (Larus ridibundus) feeding at sewage treatment works Journal of Applied Bacteriology, 56, 499-502. Fricker, C. R. & Girdwood, R. W. A. (1984). The effect of the use of different selective media on the ability to recover salmonellae from seagull faeces Journal of Hygiene, Cambridge, 93, 35-42. Fricker, C. R., Girdwood, R. W. A. & Munro, D. (1983). A comparison of enrichment media for the isolation of salmonellae from seagull cloacal swabs. Journal of Hygiene, Cambridge, 91, 53-58. Girdwood, R. W. A., Fricker, C. R., Munro, D., Shedden, C. B. & Monaghan, P. (in press). The incidence and significance of salmonella carriage by gulls (Larus spp.) in Scotland. Journal of Hygiene. Greig, S. A., Coulson, J. C. & Monaghan, P. (in press). Feeding strategies of male and female herring gulls Behaviour. Grant, P. J. (1982). Gulls: A Guide to Identification. T & A. D. Poyser Ltd., Calton. Hickling, R. A. O. (1977). The inland wintering of gulls in England & Wales. Bird Study, 24, 79-88. Horton, N., Brough, T. & Rochard, J. B. A. (1983). The importance of refuse tips to gulls wintering in an inland area of south-east England. Journal of Applied Ecology. 20, 751-765 Johnston, W. S., Maclachlan, G. K. & Hopkins, G. F. (1979). The possible involvement of seagulls (Larus sp.) in the transmission of salmonella in dairy cattle. Veterinary Record, 105, 526-527. Jones, F., Smith, P. & Watson, D. C. (1978). Pollution of a water supply catchment by breeding gulls and the potential environmental health implications Journal of Institute of Water Engineers & Scientists, 32, 469-482. Kihlman, J. & Larsson, L. (1974). On the importance of refuse dumps as a food source of wintering Herring Gulls Larus argentatus Pont. Ornis Scandinavica, 5, 63-70. MacDonald, J. W. & Brown, P. D. (1974). Salmonella infection in wild birds in Britain. Veterinary Record, 94, 321-322. Monaghan, P. (1980). Dominance and dispersal between feeding sites in the Herring Gull (Larus argentatus). Animal Behaviour, 28, 521-527. Monaghan, P. & Coulson, J. C. (1977). The status of large gulls nesting on buildings. Bird Study, 24, 89-104. Parsons, J. & Duncan, N. (1978). Recoveries and dispersal of Herring Gulls from the Isle of May. Journal of Animal Ecologv, 47, 933-1005 Reilly, W. J., Forbes, G. 1., Patterson, G. M. & Sharp, J. C. M. (1981). Human and animal salmonellosis in Scotland associated with environmental contamination, 1973-74. Veterinary Record, 108, 553-555. Shedden, C. B. (1983). Feeding and roosting behaviour of gulls: implications for water storage. Ph.D. thesis. University of Glasgow. Sibley, R. M. & McCleery, R. H. (1983). Increase in weight of herring gulls while feeding. Journal of Animal Ecology, 52, 35-50 Spaans, A. L. (1971). On the feeding ecology of the Herring Gull Larus argentatus Pont. in the northern part of the Netherlands. Ardea. 59, 73-188. Spence, J. B. & Westwood, A. (1978). Salmonella agona infection in sheep. Veterinary Record, 102, 332-336 Vernon, E. (1977). Food Poisoning in England and Wales 1973-75. Public Health, London, 81, 225-235. Williams, B. M., Richards, P. W. & Lewis, J. (1976). Salmonella infection in the herring gull (Larus argentatus). Veterinary Record. 98, 51